Description
T-Cups are designed with a top-quality durable plastic body to maintain large volume specimens for lab receiving. The TDOA-1145A3 is a non-instrumented rapid urinalysis 14-panel drug test device with adulterants and a clear view window for simple test result interpretation. The T-Cup has become known as one of the most accurate drug test cups in the marketplace and is the ideal device to be used in clinical, physician offices, laboratories, and recovery centers. With a 99% level of accuracy, each T-Cup provides quick results in only 5 minutes and includes a temperature strip to validate the specimen's temperature range between 90 - 100⁰F. The T-Cups are fast, reliable, accurate, and come with our Low Price Guarantee.
KEY FEATURES:
- FDA 510k Approved and CLIA Waived
- AMP1000, BAR300, BUP10, BZO300, COC300, MAMP1000, MDMA500, OPI300, MTD300, OXY100, PCP25, PPX300, TCA1000, THC50, CR-SG-PH
- Plastic is durable and designed to maintain sample volumes for lab receiving
- Includes adulterant strip to prevent tampering
- 24 Month Shelf-Life from date of manufacture
- Includes temperature strip (90 - 100⁰F)
- Up to 99% accurate
Urinalysis drugs of abuse assays provide only a preliminary qualitative test result. To obtain a confirmed quantitative analytical result a more specific method must be used. Gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS) are the preferred confirmatory methods. It will be important to apply clinical and professional judgment to any drugs of abuse test result, particularly when preliminary positive results are received.
PRODUCT ATTACHMENTS:
INSTRUCTIONS:
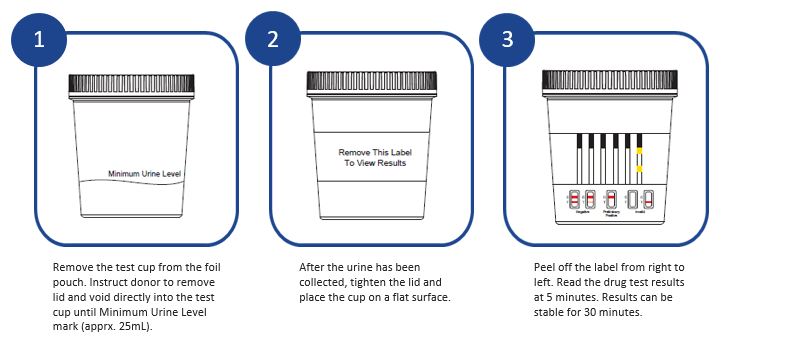
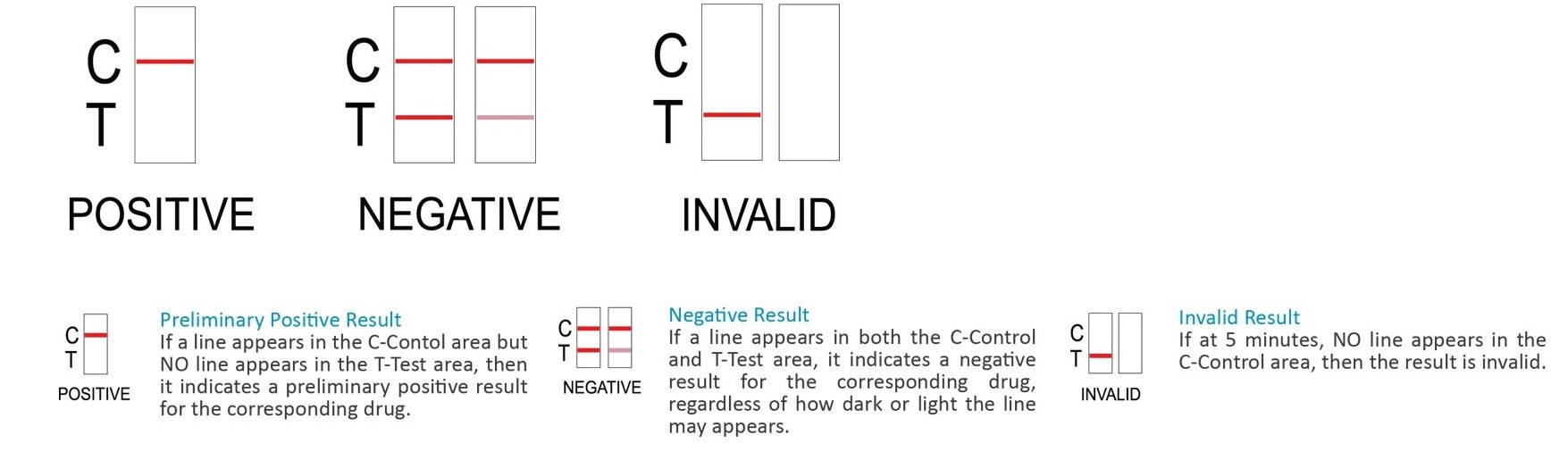
Additional Information
Classification: |
FDA Approved & CLIA Waived |
Contents: |
25 Tests/Box |
Country of Origin: |
China |
Low Price Guarantee: |
We guarantee the best price for any drug test cup in the marketplace with an identical configuration |














